 Research Article
Research Article
Characterization of Acridine Orange in Homogeneous Media: A Supportive Study and Validation of Its Potential for Photo-Applications
Flávia AP de Morais1*, Katieli Silva Souza Campanholi1, Rodolfo Bento Balbinot2 Ranulfo Combuca da Silva1, Renato S. Gonçalves1, Ana Carolina Vieira de Oliveira1, Silvio Mayke Leite3, and Wilker Caetano1
1Department of Chemistry, State University of Maringá, Brazil
1Technological Innovation Laboratory in the Pharmaceuticals and Cosmetics Development, State University of Maringá, Brazil
1Department of Animal Science, State University of Maringá, Brazil
Flávia Amanda Pedroso de Morais, Department of Chemistry, State University of Maringá, Brazil.
Received Date:November 15, 2021; Published Date:Decemebre 02,2021
Abstract
Acridine orange (AO) is a synthetic fluorescent compound that presents a series of biological applications. Although this compound is frequently used as a biological marker, only a few studies have been published regarding its intrinsic properties and its applicability as a phototherapeutic agent, thus, its physicochemical behavior in homogeneous media still is required. Therefore, in this research, we describe the physical-chemical characteristics of the AO, aiming to validate and strengthen the basic studies regarding its promising phototherapeutic potential. Using its absorption and emission spectra profile, pKa, and Kwo evaluation we verified that OA has low solubility in a homogeneous medium, presence of a positive charge at physiological pH and that it can interact both with cell membranes and with blood plasma, evidenced by its amphiphilic behavior. Other studies are still needed to validate its safety regarding the application, however, AO has many potentially favorable characteristics for phototherapeutic applications.
Keywords:Acridine orange; Photophysical properties; Homogeneous media; Phototherapeutic agent
Introduction
Theranostics is a therapeutic concept based on the combination of diagnostic and therapeutic resources in a single platform, mainly concerning cancer treatment4,8. This means that theranostic agents allow the simultaneous diagnosis and treatment of disease and allow real-time monitoring of the disease’s evolution [1]. In this way, it is possible to make the therapy more personalized, which may lead to an improvement in the prognosis of the disease. This methodology approach can be applied in several therapeutic strategies, such as chemotherapy, radiotherapy, photothermal therapy (PTT), gene therapy, and photodynamic therapy (PDT)2.
PDT approach consists of the photo-oxidation of biomolecules promoted by a photosensitizer compound which, in the presence of light with an appropriate wavelength and molecular oxygen, generates cytotoxic reactive oxygen species (ROS), as hydroxyl radicals, hydrogen peroxides, and superoxide anion, or can also cause the formation of oxygen singlet (1O2). ROS generated can react with biomolecules that trigger cell death by necrosis, apoptosis, and/or autophagy [1,2]. As it is a non-invasive, non-toxic technique, selective for diseased cells and with low side effects, photodynamic inactivation has been gaining space.
To be applicable in PDT, the photosensitizer must have some specific characteristics, which includes high light absorption in the visible region, high singlet oxygen quantum yield, low photobleaching reaction yield, high affinity and tissue penetration, favorable pharmacokinetics, non-prolonged photosensitivity, reproducibility, high stability and low toxicity in the dark [3,2,4].
3,6-bis-dimethylaminoacridine, also known as acridine orange (AO), was first synthesized in 1889, but its wide use only started from 1940-1950 due to the discovery of its ability to bind to nucleic acids [5]. Since then, a series of biological applications have been attributed to this fluorophore, and its use as an antibacterial, antiparasitic, pH indicator and photosensitizer agent has already been described [6]. The main characteristic of AO is that it is a cellpermeable dye that, after binding to dsDNA, emits green fluorescence and when it binds to RNA or ssDNA it emits red fluorescence [7,8]. AO staining is a useful and easy way to distinguish active and inactive reproductive cells, detect intracellular pH gradients, measuring apoptosis and proton pump activity [9,10,11].
AO was recently applied in cancer therapy due to its preferential accumulation in acidic environments, thus, in cancer tissue, and due to its intercalation within the DNA double helix, where the photodynamic effect induces the transition of molecular oxygen to the singlet state, which has cytotoxic activity. Evidencing AO as a promising photosensitizer for use in PDT [12-16]. However, despite the wide use of AO applied to biological sciences, its physicochemical behavior in non-aqueous solvents is quite limited. Therefore, in this research, we describe the physical-chemical characteristics of the AO, aiming to validate its promising photosensitizer properties for use in PDT, theranostic and potential therapeutic use against tumors and infections.
Materials and Methods
OA (≥ 97.0% HPLC, MM= 179.22 g mol-1) was purchased from Sigma-Aldrich and used without additional purification. All the used organic solvents (water, ethanol, octanol, and chloroform) were utilized as purchased.
OA spectroscopy characterization
Molar absorptivity was obtained using the Lambert-Beer Law at 30.0°C. The analysis was accompanied by electronic absorption spectra (Spectrophotometer Beckman DU-800) and fluorescence emission (Spectrofluorimeter Cary Eclipse) at 3.6x10-6 mol L-1 and 7.2x10-6 mol L-1, respectively. The analysis were performed using λexc= 452 nm; λemiss= 550 nm; slit 2.5/2.5, optical pass 1.00 cm and 30.0 °C.
pKa of OA in an aqueous medium
The determination of the pKa of OA (3.6x10-6 mol L-1) was performed in an aqueous medium, using Macvaine buffer, and Boric Acid buffer (5 mmol L-1), by UV-Vis electronic absorption spectroscopy, at 30.0°C. Data were evaluated by the multivariate chemometric method.
n-octanol-water partition coefficient (Kow)
Kow experiment was carried out using a water/octanol mixture (50% V/V) containing OA (1.8 x10-6 mol L-1). This mixture was subjected to vigorous stirring for 5 min and then kept at rest for 48 h in the dark. Electronic absorption spectra accessed the OA partition in water and octanol at 30.0°C. The value of Kow was calculated using Equation 1.
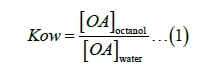
[OA]octanol is the concentration of OA in octanol, and [OA] water is the concentration of OA in water.
Results and Discussion
AO is a water-soluble fluorescent PS compound. The following analyses aim to detail the influence of the structural organization of AO on its ability to act as a diagnostic source and photosensitizer in PDT. The spectroscopic behavior is shown in Figure 1.
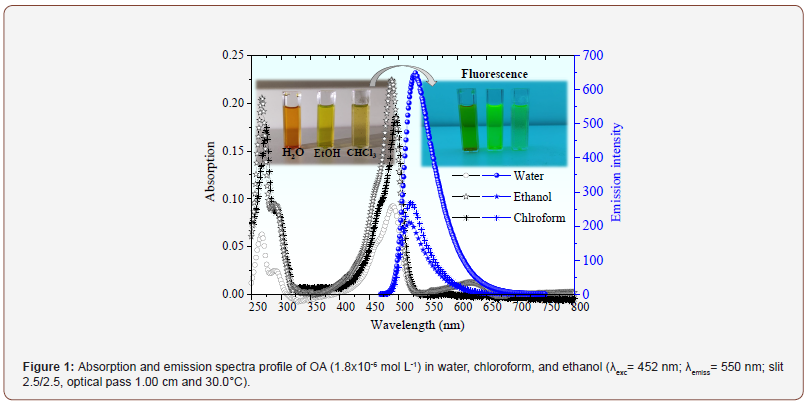
The UV-vis spectroscopy (Figure 1) showed bands relative to π-π* transition, with maximums at 265 nm, 290 nm, 470 nm, and 491 nm [10,11]. The AO has displacement in water due to hydrogen bonds with the polar and protic solvent. The peak at 491 nm is a characteristic of the AO in the monomeric state. On the other hand, its π-conjugation chain allows the formation of smaller dimers in higher polarity solvents, which results in a shoulder band at around 470 nm [17]. Aggregation tendency reflects in a lower molar absorption coefficient in water (74.8x103 cm-1 mol-1 L, Adj-R-Square 0.9914 and error of 2.8 x103), which is less expressive in ethanol (131.4 x103 cm-1 mol-1 L, Adj-R-Square 0.9778 and error of 6.0 x103) and chloroform (153.2 x103 cm-1 mol-1 L, Adj-R-Square 0.9949 and error of 4.1 x103). The molar absorption coefficient values were obtained from the absorption spectra. However, emission spectra were also considered during the analyses (selection of OA concentration that shows a linear relationship between emission intensity and drug concentration).
Furthermore, Figure 1 showed an emission band close to 520 nm. Its signal was a mirror image of the absorption spectra due to the similarity between the fundamental and excited state vibrational levels [18]. Particularly, the Stokes shift allows estimating the luminescent efficiency of the drug. In general, molecules with more significant Stokes shifts have easily detectable fluorescence, an advantage for theranostics application. The most significant shifts were obtained for AO in water (36 nm), followed by ethanol (31 nm) and chloroform (24 nm). Thus, it can be stated that although AO aggregates exist in water (aggregates commonly do not emit fluorescence), the remaining monomeric species act efficiently as a fluorescent probe. This property gives AO potential for use in theranostics. After knowing the spectroscopic properties of AO, we sought to investigate its charge state at blood pH. The results are shown in Figure 2.
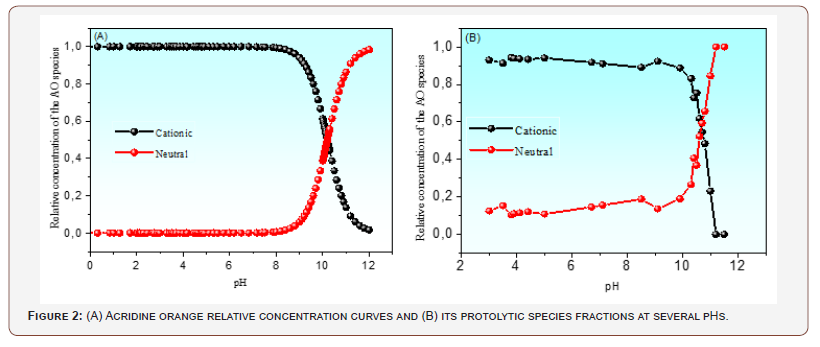
Figure 2 shows the pKa value of AO in an aqueous medium determined by the multivariate chemometric method. This method considers the contribution of the protolytic species at all wavelengths, leading to more excellent reliability of the results obtained. The relative concentrations of the protolytic species obtained chemometrically were applied to the Handerson- Hasselbalch equation, yielding a pKa of 10.7. Thus, at physiological pH, the AO molecule is expected to be in the form of AOH+. Its charge state favors interaction with cell membranes and DNA, as widely reported in the literature. Besides AO/biological system interactions of an electrostatic nature, this photosensitizer compound seems also present an amphiphilic property. This behavior was validated using the n-octanol-water partition coefficient as log Kow.
The n-octanol-water partition coefficient as log Kow is defined as the ratio of the equilibrium concentration of a dissolved compound in two-phase immiscible solvents, thus, n-octanol and water [10,12]. This method is frequently used to determine the degree of hydrophobicity by the prediction of their partitioning tendency. Kow also allows knowing this preferential location of the compound during its application, thus, the preferential partitioning of the compound to environments of lower polarity or dissolve in blood plasma [19].
The log Kow value obtained for the AO was 0.7, consistent with amphiphilic species. The evaluation of the OA amphiphilic behavior was also performed by partition kinetics. These experiments were performed monitoring both solvent phases in the presence of OA. The results are shown in Figure 3
Figure 3 shows the AO partition kinetic profile in the n-octanol phase after 10 h. It is possible to observe the AO mild preferential partition to the lower polarity solvent indicating that AO might present some interaction with membranes. The same experiment was carried out monitoring the water phase (not shown). The results confirmed the Kow value obtained and the amphiphilic behavior of this compound. Apparently, some part of AO remains in the polar solvent, thus, AO could interact with both environments without the need for a drug delivery system. These results corroborate the values of the molar absorptivity coefficient found (80.2x103 cm-1 mol-1 L, Adj-R-Square 0.9814, error of 2.9 x103 in n-octanol and 74.8x103 cm-1 mol-1 L, Adj-R-Square 0.9914, error of 2.8 x103 in water). Since these coefficients presented very close values, it is possible to state that the solubilization capacity of this compound is similar in both systems, thus, water and n-octanol, confirming its amphiphilic capacity.

Since AO is a photosensitizer compound, an excellent gross contrast agent, and an ideal contrast agent for in-vivo microscopy, without needing a formulation, these results strongly suggest its application possibility as a theranostic agent [9,10,13]. Recently, Hany Osman et al., 2018 had demonstrated the impressive potential of AO as a photosensitizer and contrast agent against glioblastoma cells [14]. The photodynamic effect was reached with almost 10 min of white light using this compound but further studies still are necessary for the AO validation as a safe drug [14]. Other studies are still needed to validate other physical-chemical properties of AO. It is also necessary some evaluations concerning its safety regarding the application, as well as some in vitro and in vivo studies with this promising compound. However, AO has many potentially favorable characteristics for phototherapeutic applications.
Conclusion
We have supported the potentially impressive effect as a photosensitizer and photodiagnostic agent of AO by describing its physical-chemical characteristics in a homogenous media. The emission and the absorption spectra profile of this compound presented similarity between the fundamental and excited state vibrational levels with lower molar absorption coefficients and significant Stokes shift in all solvents evaluated. This property allows estimating the luminescent efficiency of the drug by fluorescence, an advantage for theranostics application. Additionally, using the multivariate chemometric method we sought to investigate the AO charge state at blood pH. The results pointed to pKa of 10.7, thus, at physiological pH, the AO molecule is expected to be as AOH+, allowing its interaction with cell membranes and DNA. Although AO had presented an amphiphilic behavior (log Kwo was 0.7), thus, it can be partitioned in both environments: cell membrane and in blood plasma, further studies validating its safety photodynamic and photodiagnostic effect are warranted.
Acknowledgments
We are very thankful for the financial support from the Brazilzian Agencies for Support and Evaluation of Graduate Education: CAPES, CNPq, UGF-SETI/PR and Fundação Araucária- SETI/PR. F.A. P.d.M., R.S.G., K.S.C, N.H., and W.C. funds received from the Institutional Research Project at State University of Maringá (UEM; Grant 4588/2014-2020). F.A.P.d.M., R.S.G., N.H., and W.C. funds received from the National Council for Scientific and Technological Development (CNPq; Grant p 308142/2014-4 and 162876/2020-3). R.S.G. funds received from Coordination for the Improvement of Higher Education Personnel (CAPES; 2012-2016) [20,21].
Disclosure statement
We declare no conflicts of interest.
References
- Jenni S, Sour A (2019) Molecular Theranostic Agents for Photodynamic Therapy (PDT) and Magnetic Resonance Imaging (MRI). Inorganics 7(1): 10.
- Moghassemi S, Dadashzadeh A, Azevedo RB, Feron O, Amorim CA (2021) Photodynamic cancer therapy using liposomes as an advanced vesicular photosensitizer delivery system. Journal of Controlled Release 339: 75-90.
- García CM, Flores YM, Vázquez JM de la R, Soriano-Pérez EE, Hernández JRV, et al. (2019) Theragnosis with PDT and IgM antibody anti isoform 4 SOD mitochondrial labeled with PpIX in SiHa, MDA-MB-231 and CaCo-2 cell lines. In: AIP Conference Proceedings. American Institute of Physics Inc pp.040005.
- da Silva-Junior RC, Campanholi K da SS, de Morais FAP (2021) Photothermal Stimuli-Responsive Hydrogel Containing Safranine for Mastitis Treatment in Veterinary Using Phototherapy. ACS Applied Biomaterials 4(1): 581-596.
- Von Bertalanffy L, Bickis I (1956) Identification of Cytoplasmic Basophilia (Ribonucleic Acid) By Fluorescence Microscopy. Journal of Histochemistry & Cytochemistry 4(5): 481-493.
- Guimarães RS, Rodrigues CF, Fernandes N (2021) Combinatorial delivery of doxorubicin and acridine orange by gold core silica shell nanospheres functionalized with poly (ethylene glycol) and 4-methoxybenzamide for cancer targeted therapy. Journal of Inorganic Biochemistry 219: 111433.
- Darzynkiewicz Z (1990) Chapter 27 Differential Staining of DNA and RNA in Intact Cells and Isolated Cell Nuclei with Acridine Orange 33: 285-298.
- Yektaeian N, Mehrabani D, Sepaskhah M, Zare S, Jamhiri I, et al. (2019) Lipophilic tracer Dil and fluorescence labeling of acridine orange used for Leishmania major tracing in the fibroblast cells. Heliyon 5(12): e03073.
- Kapuscinski J a N, Melamed MR (1983) Interactions of Acridine Orange with Nucleic Acids Stranded Ribonucleic Acid. Biochem Pharmacol 32(24): 3679-3694.
- Lagutschenkov A, Dopfer O (2011) Infrared spectrum of a protonated fluorescence dye: Acridine orange. Journal of Molecular Spectroscopy 268(1-2): 66-77.
- Sharma VK, Sahare PD, Rastogi RC, Ghoshal SK, Mohan D (2003) Excited state characteristics of acridine dyes: Acriflavine and acridine orange. Spectrochimica Acta - Part A: Molecular and Biomolecular Spectroscopy 59(8): 1799-1804.
- Iessi E, Logozzi M, Lugini L (2017) Acridine Orange/exosomes increase the delivery and the effectiveness of Acridine Orange in human melanoma cells: A new prototype for theranostics of tumors. J Enzyme Inhib Med Chem 32(1): 648-657.
- Lin YC, Lin JF, Tsai TF (2017) Acridine orange exhibits photodamage in human bladder cancer cells under blue light exposure. Scientific Reports 7(1): 14103.
- Osman H, Elsahy D, Saadatzadeh MR (2018) Acridine Orange as a Novel Photosensitizer for Photodynamic Therapy in Glioblastoma. World Neurosurg 114: e1310-e1315.
- Pitchaimani A, Renganathan A, Cinthaikinian S, Premkumar K (2014) Photochemotherapeutic effects of UV-C on acridine orange in human breast cancer cells: potential application in anticancer therapy. RSC Advances 4(42): 22123.
- M Krishnamurthy B, Pal H (2021) Unraveling the salt induced modulation in the photophysical behavior of acridine orange dye on its interaction with natural DNA. Journal of Molecular Liquids 336: 116146.
- Garcia Fernandez MI, Ceccarelli D, Muscatello U (2004) Use of the fluorescent dye 10-N-nonyl acridine orange in quantitative and location assays of cardiolipin: A study on different experimental models. Anal Biochem 328(2): 174-180.
- Lakowicz JR (2006) Principles of Fluorescence Spectroscopy. US. 46312-46314
- Krop HB, van Velzen MJM, Parsons JR, Govers HAJ (1997) n-Octanol-water partition coefficients, aqueous solubilities and Henry’s law constants of fatty acid esters. Chemosphere 34(1): 107-119.
- Türker Saçan M, Inel Y (1995) Application of the characteristic root index model to the estimation of N-octanol/water partition coefficients. polychlorinated biphenyls. Chemosphere 30(1): 39-50.
- Sayed M, Krishnamurthy B, Pal H (2021) Unraveling the salt induced modulation in the photophysical behavior of acridine orange dye on its interaction with natural DNA. Journal of Molecular Liquids 336: 116146.
-
Flávia AP de Morais, Katieli Silva Souza Campanholi, Rodolfo Bento Balbinot. Characterization of Acridine Orange in Homogeneous Media: A Supportive Study and Validation of Its Potential for Photo-Applications. Arch Phar & Pharmacol Res. 3(1): 2021. APPR. MS.ID.00055
-
Acridine Orange; Photophysical Properties; Homogeneous Media; Phototherapeutic Agent.
-

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.






